植物磷脂酸的特性及其在ABA诱导气孔运动中的作用
- 中国农业科学院农业环境与可持续发展研究所国家作物高效用水与抗灾减损工程实验室,农业部旱作节水农业重点开放实验室, 北京 100081
收稿日期: 2018-05-07
录用日期: 2018-08-23
网络出版日期: 2019-09-01
基金资助
国家重点研发计划(2017YFD0201702)
Characteristics of Phosphatidic Acid and the Underlying Mechanisms of ABA-induced Stomatal Movement in Plants
- Yajing Wang ,
- Xinying Zhang ,
- Guirong Huang ,
- Xiaoying Liu ,
- Rui Guo ,
- Fengxue Gu ,
- Xiuli Zhong ,
- Xurong Mei
- Key Laboratory of Dryland Farming and Water-saving Agriculture, Ministry of Agriculture, State Engineering Laboratory of Efficient Water Use and Disaster Mitigation for Crops, Institute of Environment and Sustainable Development in Agriculture, Chinese Academy of Agricultural Sciences, Beijing 100081, China
Received date: 2018-05-07
Accepted date: 2018-08-23
Online published: 2019-09-01
摘要
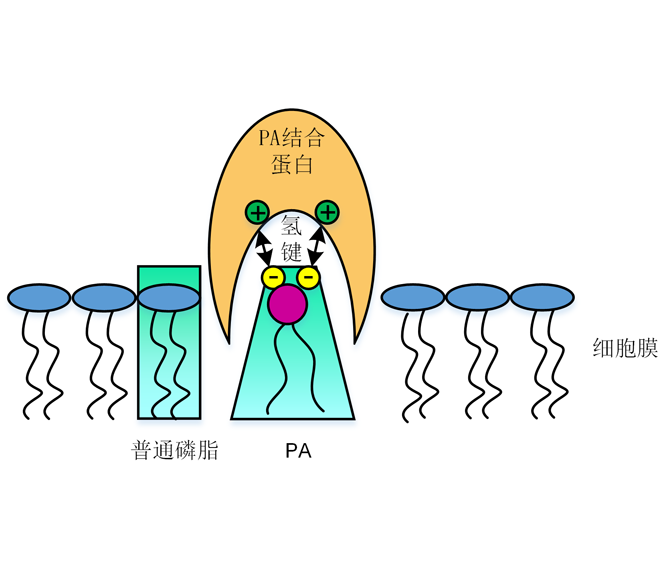
磷脂酸(PA)是应答多种生理过程的第二信使, 其作为一个脂质信号快速积累从而响应多种环境。PA主要通过磷脂酶D (PLD)和磷脂酶C/甘油二酯激酶(PLC/DGK)途径产生。基于PLDs的生化特性、激活机制以及在不同类型胁迫下被激活的特定同种型的差异, 不同类型胁迫下会产生特定分子种组成的PA。PA在多种环境下起信号转导作用, 在调节气孔运动中, PA的作用模式主要是通过与多种蛋白结合, 激活或抑制这些蛋白的活性, 进而执行其信使功能。该文主要综述PA的生化特性以及信号途径中PA互作蛋白的研究进展, 并提出PA研究中亟待解决的问题及今后的重点研究方向。
本文引用格式
王雅静 , 张欣莹 , 黄桂荣 , 刘晓英 , 郭瑞 , 顾峰雪 , 钟秀丽 , 梅旭荣 . 植物磷脂酸的特性及其在ABA诱导气孔运动中的作用[J]. 植物学报, 2019 , 54(2) : 245 -154 . DOI: 10.11983/CBB18115
Abstract
Phosphatidic acid (PA), a second messenger, is considered to be a lipid signal whose level increases transiently in response to various challenges. The main production of PA derives from phospholipase D (PLD) and phospholipase C/diacylglycerol kinase (PLC/DGK) pathways. On the basis of differences in biochemical properties, catalytic mechanisms, and heterogeneities of PLDs, which are activated under specific stress, diverse PA molecular species composition would be formed response to various stresses conditions. PA is involved in various physiological processes. It acts as messenger by binding target proteins and regulating proteins positively and negatively in stomatal closure pathways. In this review, we first summarize biochemical properties of PA and the effects of PA on protein interaction and then provide insights into some crucial and urgent issues as well as directions for future research.

Key words: cytoskeleton; mechanism; phosphatidic acid; signal transduction; stomatal movement
参考文献
| [1] | 李莉, 井文, 章文华 ( 2015). 植物细胞中磷酸肌醇和磷脂酶C介导的信号转导. 植物生理学报 51, 1590-1596. |
| [2] | 李一路, 张晴晴, 胡卫芹, 屈钢, 洪月云 ( 2017). 磷脂酸磷酸酶在脂质代谢和信号转导中的作用及其调控. 植物生理学报 53, 897-904. |
| [3] | 王涛, 梅旭荣, 钟秀丽, 李玉中, 曾正兵, 王海燕, 孙磊, 夏旭 ( 2010). 磷脂酶Dδ参与植物的低温驯化过程. 植物学报 45, 541-547. |
| [4] | Barenholz Y, Gibbes D, Litman BJ, Goll J, Thompson TE, Carlson FD ( 1977). A simple method for the preparation of homogeneous phospholipid vesicles. Biochemistry 16, 2806-2810. |
| [5] | Bargmann BOR, Laxalt AM, Riet BT, Schouten E, van Leeuwen W, Dekker HL, de Koster CG, Haring MA, Munnik T ( 2006). LePLDβ1 activation and relocalization in suspension-cultured tomato cells treated with xylanase. Plant J 45, 358-368. |
| [6] | Bargmann BOR, Laxalt AM, ter Riet B, van Schooten B, Merquiol E, Testerink C, Haring MA, Bartels D, Munnik T ( 2009). Multiple PLDs required for high salinity and water deficit tolerance in plants. Plant Cell Physiol 50, 78-89. |
| [7] | Chalfant CE, Spiegel S ( 2005). Sphingosine 1-phosphate and ceramide 1-phosphate: expanding roles in cell signaling. J Cell Sci 118, 4605-4612. |
| [8] | Cruz-Ramirez A, Oropeza-Aburto A, Razo-Hernández F, Ramírez-Chávez E, Herrera-Estrella L ( 2006). Phospholipase DZ2 plays an important role in extraplastidic galactolipid biosynthesis and phosphate recycling in Ara- bidopsis roots. Proc Natl Acad Sci USA 103, 6765-6770. |
| [9] | Devaiah SP, Pan XQ, Hong YY, Roth M, Welti R, Wang XM ( 2007). Enhancing seed quality and viability by suppres- sing phospholipase D in Arabidopsis. Plant J 50, 950-957. |
| [10] | Distéfano AM, Valiñas MA, Scuffi D, Lamattina L, ten Have A, García-Mata C, Laxalt AM ( 2015). Phospholipase D δ knock-out mutants are tolerant to severe drought stress. Plant Signal Behav 10, e1089371. |
| [11] | Fan L, Zheng SQ, Wang XM ( 1997). Antisense suppression of phospholipase Dα retards abscisic acid- and ethy- lene-promoted senescence of postharvest Arabidopsis leaves. Plant Cell 9, 2183-2196. |
| [12] | Finkelstein RR, Gampala SSL, Rock CD ( 2002). Abscisic acid signaling in seeds and seedlings. Plant Cell 14, S15-S45. |
| [13] | Gosti F, Beaudoin N, Serizet C, Webb AAR, Vartanian N, Giraudat J ( 1999). ABI1 protein phosphatase 2C is a negative regulator of abscisic acid signaling. Plant Cell 11, 1897-1909. |
| [14] | Guo L, Mishra G, Markham JE, Li MY, Tawfall A, Welti R, Wang XM ( 2012). Connections between sphingosine kinase and phospholipase D in the abscisic acid signaling pathway in Arabidopsis. J Biol Chem 287, 8286-8296. |
| [15] | Guo L, Mishra G, Taylor K, Wang XM ( 2011). Phosphatidic acid binds and stimulates Arabidopsis sphingosine kina- ses. J Biol Chem 286, 13336-13345. |
| [16] | Himmelbach A, Hoffmann T, Leube M, Höhener B, Grill E ( 2002). Homeodomain protein ATHB6 is a target of the protein phosphatase ABI1 and regulates hormone responses in Arabidopsis. EMBO J 21, 3029-3038. |
| [17] | Hong YY, Devaiah SP, Bahn SC, Thamasandra BN, Li MY, Welti R, Wang XM ( 2009). Phospholipase Dε and phosphatidic acid enhance Arabidopsis nitrogen signaling and growth. Plant J 58, 376-387. |
| [18] | Hong YY, Pan XQ, Welti R, Wang XM ( 2008a). Phospholipase Dα3 is involved in the hyperosmotic response in Arabidopsis. Plant Cell 20, 803-816. |
| [19] | Hong YY, Zhang WH, Wang XM ( 2010). Phospholipase D and phosphatidic acid signaling in plant response to drought and salinity. Plant Cell Environ 33, 627-635. |
| [20] | Hong YY, Zhao J, Guo L, Kim SC, Deng XJ, Wang GL, Zhang GY, Li MY, Wang XM ( 2016). Plant phospholipases D and C and their diverse functions in stress responses. Prog Lipid Res 62, 55-74. |
| [21] | Hong YY, Zheng SQ, Wang XM ( 2008b). Dual functions of phospholipase Dα1 in plant response to drought. Mol Plant 1, 262-269. |
| [22] | Jang JH, Lee CS, Hwang D, Ryu SH ( 2012). Understanding of the roles of phospholipase D and phosphatidic acid through their binding partners. Prog Lipid Res 51, 71-81. |
| [23] | Jiang Y, Wu K, Lin F, Qu YN, Liu XX, Zhang Q ( 2014). Phosphatidic acid integrates calcium signaling and microtubule dynamics into regulating ABA-induced stomatal closure in Arabidopsis. Planta 239, 565-575. |
| [24] | Kalachova T, Iakovenko O, Kretinin S, Kravets V ( 2013). Involvement of phospholipase D and NADPH-oxidase in salicylic acid signaling cascade. Plant Physiol Biochem 66, 127-133. |
| [25] | Katagiri T, Takahashi S, Shinozaki K ( 2001). Involvement of a novel Arabidopsis phospholipase D, AtPLDδ, in dehydration-inducible accumulation of phosphatidic acid in stress signaling. Plant J 26, 595-605. |
| [26] | Kennedy EP ( 1958). The biosynthesis of phospholipids. Am J Clin Nutr 6, 216-220. |
| [27] | Klimecka M, Szczegielniak J, Godecka L, Lewandowska- Gnatowska E, Dobrowolska G, Muszynska G ( 2011). Regulation of wound-responsive calcium-dependent protein kinase from maize (ZmCPK11) by phosphatidic acid. Acta Biochim Pol 58, 589-595. |
| [28] | Kolesnikov YS, Nokhrina KP, Kretynin SV, Volotovski ID, Martinec J, Romanov GA, Kravets VS ( 2012). Molecular structure of phospholipase D and regulatory mechanisms of its activity in plant and animal cells. Biochemistry (Mosc) 77, 1-14. |
| [29] | Kooijman EE, Chupin V, de Kruijff B, Burger KNJ ( 2003). Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic 4, 162-174. |
| [30] | Kooijman EE, Tieleman DP, Testerink C, Munnik T, Rijkers DTS, Burger KNJ, de Kruijff B ( 2007). An electrostatic/hydrogen bond switch as the basis for the specific interaction of phosphatidic acid with proteins. J Biol Chem 282, 11356-11364. |
| [31] | Lee J, Welti R, Roth M, Schapaugh WT, Li JR, Trick HN ( 2012). Enhanced seed viability and lipid compositional changes during natural ageing by suppressing phospholipase Dα in soybean seed. Plant Biotechnol J 10, 164-173. |
| [32] | Li MY, Hong YY, Wang XM ( 2009). Phospholipase D- and phosphatidic acid-mediated signaling in plants. Biochim Biophys Acta 1791, 927-935. |
| [33] | Li MY, Qin CB, Welti R, Wang XM ( 2006a). Double knockouts of phospholipases Dζ1 and Dζ2 in Arabidopsis affect root elongation during phosphate-limited growth but do not affect root hair patterning. Plant Physiol 140, 761-770. |
| [34] | Li MY, Welti R, Wang XM ( 2006b). Quantitative profiling of Arabidopsis polar glycerolipids in response to phosphorus starvation. Roles of Phospholipases Dζ1 and Dζ2 in phosphatidylcholine hydrolysis and digalactosyldiacylgly- cerol accumulation in phosphorus-starved plants. Plant Physiol 142, 750-761. |
| [35] | Lindeboom JJ, Nakamura M, Hibbel A, Shundyak K, Gutierrez R, Ketelaar T, Emons AMC, Mulder BM, Kirik V, Ehrhardt DW ( 2013). A Mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science 342, 1245533. |
| [36] | Mishra G, Zhang WH, Deng F, Zhao J, Wang XM ( 2006). A bifurcating pathway directs abscisic acid effects on stoma- tal closure and opening in Arabidopsis. Science 312, 264-266. |
| [37] | Munnik T, Meijer HJG, ter Riet B, Hirt H, Frank W, Bartels D, Musgrave A ( 2000). Hyperosmotic stress stimulates phospholipase D activity and elevates the levels of phosphatidic acid and diacylglycerol pyrophosphate. Plant J 22, 147-154. |
| [38] | Nomikos M, Mulgrew-Nesbitt A, Pallavi P, Mihalyne G, Zaitseva I, Swann K, Lai FA, Murray D, McLaughlin S ( 2007). Binding of phosphoinositide-specific phospholipase C-ζ (PLC-ζ) to phospholipid membranes: potential role of an unstructured cluster of basic residues. J Biol Chem 282, 16644-16653. |
| [39] | Ohashi Y, Oka A, Rodrigues-Pousada R, Possenti M, Ruberti I, Morelli G, Aoyama T ( 2003). Modulation of phospholipid signaling by GLABRA2 in root-hair pattern formation. Science 300, 1427-1430. |
| [40] | Ohlrogge J, Browse J ( 1995). Lipid biosynthesis. Plant Cell 7, 957-970. |
| [41] | Pinosa F, Buhot N, Kwaaitaal M, Fahlberg P, Thordal- Christensen H, Ellerstrom M, Andersson MX ( 2013). Arabidopsis phospholipase dδ is involved in basal defense and nonhost resistance to powdery mildew fungi. Plant Physiol 163, 896-906. |
| [42] | Pleskot R, Li JJ, Žárský V, Potocký M, Staiger CJ ( 2013). Regulation of cytoskeletal dynamics by phospholipase D and phosphatidic acid. Trends Plant Sci 18, 496-504. |
| [43] | Pleskot R, Pejchar P, Staiger CJ, Potocky M ( 2014). When fat is not bad: the regulation of actin dynamics by phospholipid signaling molecules. Front Plant Sci 5, 5. |
| [44] | Pleskot R, Potocký M, Pejchar P, Linek J, Bezvoda R, Martinec J, Valentová O, Novotná Z, Žárský V ( 2010). Mutual regulation of plant phospholipase D and the actin cytoskeleton. Plant J 62, 494-507. |
| [45] | Puli MR, Rajsheel P, Aswani V, Agurla S, Kuchitsu K, Raghavendra AS ( 2016). Stomatal closure induced by phytosphingosine-1-phosphate and sphingosine-1-phosp- hate depends on nitric oxide and pH of guard cells in Pisum sativum . Planta 244, 831-841. |
| [46] | Qin CB, Wang CX, Wang XM ( 2002). Kinetic analysis of Arabidopsis phospholipase Dδ. Substrate preference and mechanism of activation by Ca 2+ and phosphatidylinositol 4,5-bisphosphate . J Biol Chem 277, 49685-49690. |
| [47] | Rizzo MA, Shome K, Watkins SC, Romero G ( 2000). The recruitment of Raf-1 to membranes is mediated by direct interaction with phosphatidic acid and is independent of association with Ras. J Biol Chem 275, 23911-23918. |
| [48] | Ryu SB, Wang XM ( 1998). Increase in free linolenic and linoleic acids associated with phospholipase D-mediated hydrolysis of phospholipids in wounded castor bean lea- ves. Biochim Biophys Acta 1393, 193-202. |
| [49] | Shin JJ, Loewen CJ ( 2011). Putting the pH into phosphatidic acid signaling. BMC Biol 9, 85. |
| [50] | Testerink C, Larsen PB, van der Does D, van Himbergen JAJ, Munnik T ( 2007). Phosphatidic acid binds to and inhibits the activity of Arabidopsis CTR1. J Exp Bot 58, 3905-3914. |
| [51] | Testerink C, Munnik T ( 2005). Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci 10, 368-375. |
| [52] | Testerink C, Munnik T ( 2011). Molecular, cellular, and phy- siological responses to phosphatidic acid formation in plants. J Exp Bot 62, 2349-2361. |
| [53] | Torres MA, Dangl JL, Jones JDG ( 2002). Arabidopsis gp91 phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99, 517-522. |
| [54] | van den Brink-van Der Laan E, Killian JA, de Kruijff B ( 2004). Nonbilayer lipids affect peripheral and integral membrane proteins via changes in the lateral pressure profile. Biochim Biophys Acta 1666, 275-288. |
| [55] | Wang XM, Devaiah SP, Zhang WH, Welti R ( 2006). Signaling functions of phosphatidic acid. Prog Lipid Res 45, 250-278. |
| [56] | Welti R, Li WQ, Li MY, Sang YM, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang XM ( 2002). Profiling membrane lipids in plant stress responses. Role of phospholipase Dα in freezing-induced lipid changes in Arabidopsis. J Biol Chem 277, 31994-32002. |
| [57] | Worrall D, Liang YK, Alvarez S, Holroyd GH, Spiegel S, Panagopulos M, Gray JE, Hetherington AM ( 2008). Involvement of sphingosine kinase in plant cell signaling. Plant J 56, 64-72. |
| [58] | Yamaguchi T, Kuroda M, Yamakawa H, Ashizawa T, Hirayae K, Kurimoto L, Shinya T, Shibuya N ( 2009). Suppression of a phospholipase D gene, OsPLDβ1 , activates defense responses and increases disease resistance in rice. Plant Physiol 150, 308-319. |
| [59] | Yu LJ, Nie JN, Cao CY, Jin YK, Yan M, Wang FZ, Liu J, Xiao Y, Liang YH, Zhang WH ( 2010). Phosphatidic acid mediates salt stress response by regulation of MPK6 in Arabidopsis thaliana . New Phytol 188, 762-773. |
| [60] | Zhang Q, Lin F, Mao TL, Nie JN, Yan M, Yuan M, Zhang WH ( 2012). Phosphatidic acid regulates microtubule organization by interacting with MAP65-1 in response to salt stress in Arabidopsis. Plant Cell 24, 4555-4576. |
| [61] | Zhang WH, Qin CB, Zhao J, Wang XM ( 2004). Phospholipase Dα1-derived phosphatidic acid interacts with ABI1 phosphatase 2C and regulates abscisic acid signaling. Proc Natl Acad Sci USA 101, 9508-9513. |
| [62] | Zhang YY, Zhu HY, Zhang Q, Li MY, Yan M, Wang R, Wang LL, Welti R, Zhang WH, Wang XM ( 2009). Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 21, 2357-2377. |
| [63] | Zhao J, Devaiah SP, Wang CX, Li MY, Welti R, Wang XM ( 2013). Arabidopsis phospholipase Dβ1 modulates defense responses to bacterial and fungal pathogens. New Phytol 199, 228-240. |
| [64] | Zhao J, Wang CX, Bedair M, Welti R, Sumner LW, Baxter I, Wang XM ( 2011). Suppression of phospholipase Dγs confers increased aluminum resistance in Arabidopsis thaliana . PLoS One 6, e28086. |