

万寿菊花冠高效瞬时转化体系的建立及TeCYC2c基因启动子活性分析
收稿日期: 2024-10-02
录用日期: 2025-01-20
网络出版日期: 2025-01-21
基金资助
国家自然科学基金(32172616)
Establishment of an Efficient Transient Transformation System for Tagetes erecta Corollas and Analysis on the Promoter Activity of TeCYC2c Gene
Received date: 2024-10-02
Accepted date: 2025-01-20
Online published: 2025-01-21
为建立万寿菊(Tagetes erecta)花冠高效瞬时转化体系并探究花器官对称性形成关键调控基因TeCYC2c的启动子活性, 将CaMV35S启动子与GUS基因的融合表达载体瞬时转化万寿菊花冠, 探究菌株类型、菌液浓度、侵染时间和共培养时间对GUS基因瞬时转化效率的影响。结果显示, GV3101菌株的侵染效率最高; 菌液浓度OD600=1.0时转化效率最高; 侵染时间对瞬时转化效率无显著影响; 共培养1天为最佳培养时间。基于建立的万寿菊花冠瞬时转化体系, 对TeCYC2c基因的启动子活性进行探究。克隆了TeCYC2c基因上游1 735 bp序列, 并通过PlantCARE预测的元件分布位置及数量构建了4个启动子缺失表达载体, 以GUS基因为报告基因, 瞬时转化万寿菊花冠进行不同长度启动子活性研究。结果显示, 启动子核心区域位于ATG上游-650 - -1 bp, 推测该区域内的光响应元件正调控下游基因的表达, 而pTeCYC2c (-1 735)和pTeCYC2c (-1 406)所特有的植物激素响应和逆境响应元件可能具有抑制启动子驱动下游基因表达的功能。结合万寿菊花冠瞬时转化体系的建立和TeCYC2c启动子活性分析, 可为进一步快速解析花发育相关基因的功能奠定技术基础。

窦淋琳 , 朱钰 , 刘翠翠 , 臧运平 , 陶正国 , 包满珠 , 何燕红 . 万寿菊花冠高效瞬时转化体系的建立及TeCYC2c基因启动子活性分析[J]. 植物学报, 2025 , 60(6) : 875 -887 . DOI: 10.11983/CBB24150
INTRODUCTION: Marigold (Tagetes erecta), an important ornamental and medicinal plant, has a unique capitulum characteristic of the Asteraceae family with distinct ray and disc florets. However, the lack of efficient genetic transformation system has limited the research on the mechanism of floral organ development of marigold. Floral transient transformation system offers a rapid approach to study the function of genes expressed specifically in floral organs. This study aimed to establish an efficient transient transformation system for marigold corollas and to analyze the promoter activity of TeCYC2c, which highly expressed in corollas, thereby laying the technical foundation for the rapid verification of floral gene function.
RATIONALE: A fusion expression vector, incorporating the CaMV35S promoter and the GUS reporter gene, was constructed to facilitate the transient transformation in marigold corollas. The study delved into the effects of bacterial strain type (GV3101, LBA4404, EHA105), bacterial suspension concentration (OD600 values 0.5-2.0), infection duration (10- 40 min), and co-culture time (1-4 d) on the transient transformation efficiency of the GUS gene. Based on this transient transformation system for the marigold corollas, the promoter activity of the TeCYC2c gene was investigated. A 1 735 bp upstream sequence of the TeCYC2c gene was cloned and four promoter deletion expression vectors, with the GUS gene as the reporter gene, were constructed based on the distribution and quantity of elements predicted by PlantCARE. Subsequently, these vectors were employed for transient transformation of marigold corollas to facilitate an in-depth analysis of promoter activity.
RESULTS: The transient transformation efficiency in marigold corollas demonstrated that the GV3101 strain achieved the highest infection efficiency; the bacterial suspension concentration, quantified at an OD600 value of 1.0, yielded the most robust transformation efficiency; the infection time was observed to exert no substantial influence on transient transformation efficacy; moreover, a co-culture time of 24 hours was identified as the optimal condition for the process. The results of GUS staining and GUS activity assay revealed that the core region of the promoter was located at -650 to -1 bp. It was speculated that the light-responsive elements within this region positively up-regulated the expression of downstream genes, while the hormone-responsive and stress-responsive elements unique to pTeCYC2c (-1 735) and pTeCYC2c (-1 406) might have an inhibitory effect on promoter-driven downstream gene expression.
CONCLUSION: This study established an efficient transient transformation system for marigold corollas, optimized through strain selection and parameter tuning. The identification of the TeCYC2c promoter core region (-650 to -1 bp) and its regulatory elements provides critical insights into the regulatory mechanism of the TeCYC2c gene. The transient transformation system and promoter analysis method lay a technical foundation for accelerating functional studies of floral development genes in marigold, with potential applications in the genetic improvement of ornamental plants.
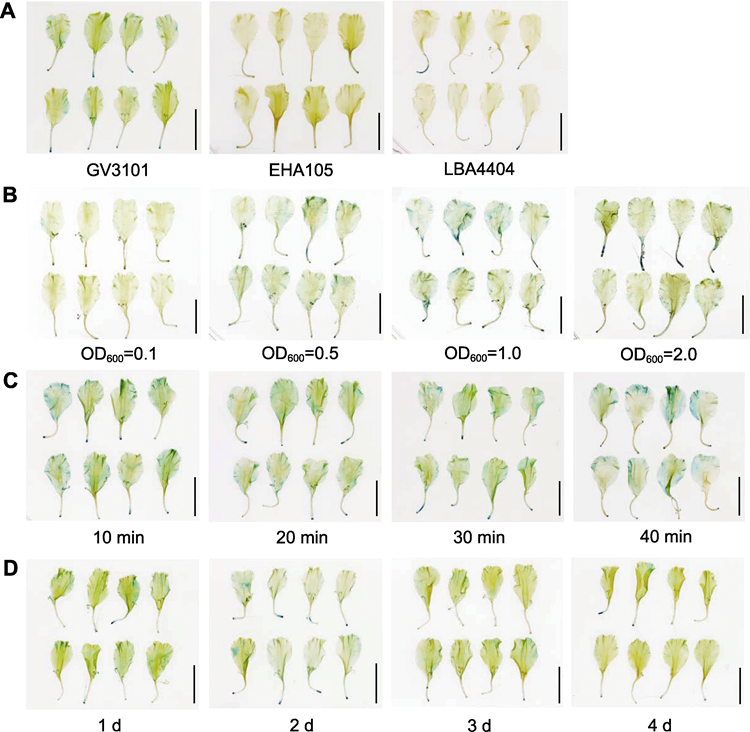
GUS staining of marigold (Tagetes erecta) corollas under different transient transformation conditions. (A) GUS staining of marigold corollas infected by three different bacterial strains; (B) GUS staining under four different concentrations (OD600) of bacterial suspension; (C) GUS staining under four different infection duration; (D) GUS staining under four different co-culture time. (A)-(D) Bars=1 cm

Key words: marigold (Tagetes erecta); TeCYC2c; promoter; transient transformation; GUS activity
| [1] | Ai Y, Zhang QH, Wang WN, Zhang CL, Cao Z, Bao MZ, He YH (2016). Transcriptomic analysis of differentially expressed genes during flower organ development in genetic male sterile and male fertile Tagetes erecta by digital gene-expression profiling. PLoS One 11, e0150892. |
| [2] | Broholm SK, T?htiharju S, Laitinen RAE, Albert VA, Teeri TH, Elomaa P (2008). A TCP domain transcription factor controls flower type specification along the radial axis of the Gerbera (Asteraceae) inflorescence. Proc Natl Acad Sci USA 105, 9117-9122. |
| [3] | Fambrini M, Pugliesi C (2017). Mobilization of the Tetu1 transposable element of Helianthus annuus: evidence for excision in different developmental stages. Biol Plant 61, 55-63. |
| [4] | Fukuda N, Ajima C, Yukawa T, Olsen JE (2016). Antagonistic action of blue and red light on shoot elongation in petunia depends on gibberellin, but the effects on flowering are not generally linked to gibberellin. Environ Exp Bot 121, 102-111. |
| [5] | Godoy-Hernández G, Berzunza EA, Concha LC, de Lourdes Miranda-Ham M (2006). Agrobacterium-mediated transient transformation of marigold (Tagetes erecta). Plant Cell Tissue Organ Cult 84, 365-368. |
| [6] | Goto N, Pharis RP (1999). Role of gibberellins in the development of floral organs of the gibberellin-deficient mutant, ga1-1, of Arabidopsis thaliana. Can J Bot 77, 944-954. |
| [7] | He YH (2010). Genetic Analysis of the Male Sterility of Tagetes erecta and Its Application in Breeding. Doctoral dissertation. Wuhan: Huazhong Agricultural University. pp. 148. (in Chinese) |
| 何燕红 (2010). 万寿菊雄性不育性状的遗传分析及其育种应用. 博士论文. 武汉: 华中农业大学. pp. 148. | |
| [8] | Hileman LC (2014). Bilateral flower symmetry-how, when and why? Curr Opin Plant Biol 17, 146-152. |
| [9] | Howarth DG, Donoghue MJ (2006). Phylogenetic analysis of the “ECE” (CYC/TB1) clade reveals duplications predating the core eudicots. Proc Natl Acad Sci USA 103, 9101-9106. |
| [10] | Hu JH, Mitchum MG, Barnaby N, Ayele BT, Ogawa M, Nam E, Lai WC, Hanada A, Alonso JM, Ecker JR, Swain SM, Yamaguchi S, Kamiya Y, Sun TP (2008). Potential sites of bioactive gibberellin production during reproductive growth in Arabidopsis. Plant Cell 20, 320-336. |
| [11] | Huang D, Li XW, Sun M, Zhang TX, Pan HT, Cheng TR, Wang J, Zhang QX (2016). Identification and characterization of CYC-like genes in regulation of ray floret development in Chrysanthemum morifolium. Front Plant Sci 7, 1633. |
| [12] | Ishiguro S, Kawai-Oda A, Ueda J, Nishida I, Okada K (2001). The DEFECTIVE IN ANTHER DEHISCENCE1 gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 13, 2191-2209. |
| [13] | Jefferson RA, Kavanagh TA, Bevan MW (1987). GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6, 3901-3907. |
| [14] | Juntheikki-Palovaara I, T?htiharju S, Lan TY, Broholm SK, Rijpkema AS, Ruonala R, Kale L, Albert VA, Teeri TH, Elomaa P (2014). Functional diversification of duplicated CYC2 clade genes in regulation of inflorescence development in Gerbera hybrida (Asteraceae). Plant J 79, 783-796. |
| [15] | Mo?nne-Loccoz Y, Mavingui P, Combes C, Normand P, Steinberg C (2015). Microorganisms and biotic interactions. In: Bertrand JC, Caumette P, Lebaron P, Matheron R, Normand P, Sime-Ngando T, eds. Environmental Microbiology: Fundamentals and Applications. Dordrecht: Springer. pp. 395-444. |
| [16] | Rieu I, Ruiz-Rivero O, Fernandez-Garcia N, Griffiths J, Powers SJ, Gong F, Linhartova T, Eriksson S, Nilsson O, Thomas SG, Phillips AL, Hedden P (2008). The gibberellin biosynthetic genes AtGA20ox1 and AtGA20ox2 act, partially redundantly, to promote growth and development throughout the Arabidopsis life cycle. Plant J 53, 488-504. |
| [17] | Su WR, Huang KL, Shen RS, Chen WS (2002). Abscisic acid affects floral initiation in Polianthes tuberosa. J Plant Physiol 159, 557-559. |
| [18] | Sun M, Yang HH, Wang AQ, Zhang YJ, Da XW, Zhang J, Sun K, Wu JP, Feng HQ (2023). Effects of different vectors and Agrobacterium tumefaciens on transient expression of alfalfa. Bull Bot Res 43, 835-845. (in Chinese) |
| 孙敏, 杨红红, 王安琪, 张悦婧, 达晓伟, 张继, 孙坤, 吴建平, 冯汉青 (2023). 不同载体和农杆菌对苜蓿瞬时表达影响的研究. 植物研究 43, 835-845. | |
| [19] | Tahtiharju S, Rijpkema AS, Vetterli A, Albert VA, Teeri TH, Elomaa P (2012). Evolution and diversification of the CYC/TB1 gene family in Asteraceae—a comparative study in gerbera (Mutisieae) and sunflower (Heliantheae). Mol Biol Evol 29, 1155-1166. |
| [20] | Tanimoto S, Miyazaki A, Harada H (1985). Regulation by abscisic acid of in vitro flower formation in Torenia stem segments. Plant Cell Physiol 26, 675-682. |
| [21] | Tong Z, Wang T, Xu Y (1990). Evidence for involvement of phytochrome regulation in male sterility of a mutant of Oryza sativa L. Photochem Photobiol 52, 161-164. |
| [22] | van der Meer IM, Spelt CE, Mol JNM, Stuitje AR (1990). Promoter analysis of the chalcone synthase (chsA) gene of Petunia hybrida: a 67 bp promoter region directs flower- specific expression. Plant Mol Biol 15, 95-109. |
| [23] | Wang RP, Liu Q, Yang ZQ, Zhang BK, Huang Z, Niu XL (2023). Cloning and analysis of carotene hydroxylase gene TeCHYE in Tagetes erecta L. J Hefei Univ Technol (Nat Sci) 46, 535-540. (in Chinese) |
| 王瑞鹏, 刘茜, 杨智强, 张博昆, 黄姿, 牛向丽 (2023). 万寿菊胡萝卜素羟化酶基因TeCHYE克隆与分析. 合肥工业大学学报(自然科学版) 46, 535-540. | |
| [24] | Wang WJ, Zhu Y, Zhang HM, Wei LD, Yi QP, Yu XM, Liu YH, Zhang LX, Cheng WH, He YH (2023). Morphological identification and development of linkage markers for lobed ray floret mutants in marigold (Tagetes erecta). Chin Bull Bot 58, 893-904. (in Chinese) |
| 王文静, 朱钰, 张洪铭, 韦陆丹, 易庆平, 余晓敏, 刘雨菡, 张莉雪, 程文翰, 何燕红 (2023). 万寿菊舌状花花冠裂片突变体的形态鉴定及连锁标记开发. 植物学报 58, 893-904. | |
| [25] | Wang YQ, Wei LD, Wang WJ, Liu BJ, Zhang CL, Zhang JW, He YH (2020). The establishment and optimization of a regeneration system for marigold (Tagetes erecta). Chin Bull Bot 55, 749-759. (in Chinese) |
| 王亚琴, 韦陆丹, 王文静, 刘宝骏, 张春玲, 张俊卫, 何燕红 (2020). 万寿菊再生体系的建立及优化. 植物学报 55, 749-759. | |
| [26] | Wen Y, Li B, Zhao DG, Zhao YC (2020). Cloning and expression analysis of the promoter of glycosyltransferase gene in Eucommia ulmoides. J Agr Biotechnol 28, 223-231. (in Chinese) |
| 文永, 李彪, 赵德刚, 赵懿琛 (2020). 杜仲糖基转移酶基因的启动子克隆及表达分析. 农业生物技术学报 28, 223-231. | |
| [27] | Yang F (2011). Establishment of PSY Plant Expression Vector Harboring Two T-DNAs and Agrobacterium-mediated Transformation System and Optimization in SSR- PCR Reaction System for Tagetes erecta L. Master’s thesis. Shanghai: Shanghai Jiao Tong University. pp. 58. (in Chinese) |
| 杨帆 (2011). 色素万寿菊PSY双边界载体及遗传转化体系建立和SSR-PCR体系的优化. 硕士论文. 上海: 上海交通大学. pp. 58. | |
| [28] | Yu XM, Wang YQ, Liu YH, Yi QP, Cheng WH, Zhu Y, Duan F, Zhang LX, He YH (2023). Establishment of Agrobacterium tumefaciens-mediated genetic transformation system of marigold (Tagetes erecta). Chin Bull Bot 58, 760-769. (in Chinese) |
| 余晓敏, 王亚琴, 刘雨菡, 易庆平, 程文翰, 朱钰, 段枫, 张莉雪, 何燕红 (2023). 根癌农杆菌介导万寿菊遗传转化体系的建立. 植物学报 58, 760-769. | |
| [29] | Yuan CQ, Huang D, Yang Y, Sun M, Cheng TR, Wang J, Pan HT, Zhang QX (2020). CmCYC2-like transcription factors may interact with each other or bind to the promoter to regulate floral symmetry development in Chrysanthemum morifolium. Plant Mol Biol 103, 159-171. |
| [30] | Zhang B (2012). The Study of Karyotype on Genus Tagetes L. and Factors in the Genetic Transformation System of psy Gene for Tagetes erecta L. Master’s thesis. Shanghai: Shanghai Jiao Tong University. pp. 64. (in Chinese) |
| 张嫔 (2012). 万寿菊属植物染色体核型分析及万寿菊psy基因遗传转化体系影响因素的研究. 硕士论文. 上海: 上海交通大学. pp. 64. | |
| [31] | Zheng L, Liu GF, Meng XN, Li YB, Wang YC (2012). A versatile Agrobacterium-mediated transient gene expression system for herbaceous plants and trees. Biochem Genet 50, 761-769. |
| [32] | Zhu Y, Liu YH, Wang WJ, Li H, Liu CC, Dou LL, Wei LD, Cheng WH, Bao MZ, Yi QP, He YH (2023). Identification and characterization of CYC2-like genes related to floral symmetric development in Tagetes erecta (Asteraceae). Gene 889, 147804. |
/
| 〈 |
|
〉 |