

Chinese Bulletin of Botany ›› 2016, Vol. 51 ›› Issue (5): 684-690.DOI: 10.11983/CBB16008
Previous Articles Next Articles
Qun Zhang1,2†, Xiuli Lü1†, Xiaoli He1, Yi Zhu1, Xinhong Cui1*
Received:2016-01-13
Accepted:2016-05-12
Online:2016-09-01
Published:2016-09-27
Contact:
Zhang Qun,Lü Xiuli,Cui Xinhong
About author:# Co-first authors
Qun Zhang, Xiuli Lü, Xiaoli He, Yi Zhu, Xinhong Cui. A Rapid Propagation System for Scirpus × mariqueter[J]. Chinese Bulletin of Botany, 2016, 51(5): 684-690.
| Treatment | No. of contamination | No. of aseptic seedlings | Percentage of germination (%) |
|---|---|---|---|
| Intact seed | 1.00±1.00 | 5.33 | 5.33b |
| Piercing naked seed | 26.00±2.65 | 15.00 | 15.00a |
| Naked seed | 38.00±3.00 | 4.00 | 4.00c |
Table 1 Germination condition of Scirpus × mariqueter seeds on M1 medium under different treatments
| Treatment | No. of contamination | No. of aseptic seedlings | Percentage of germination (%) |
|---|---|---|---|
| Intact seed | 1.00±1.00 | 5.33 | 5.33b |
| Piercing naked seed | 26.00±2.65 | 15.00 | 15.00a |
| Naked seed | 38.00±3.00 | 4.00 | 4.00c |
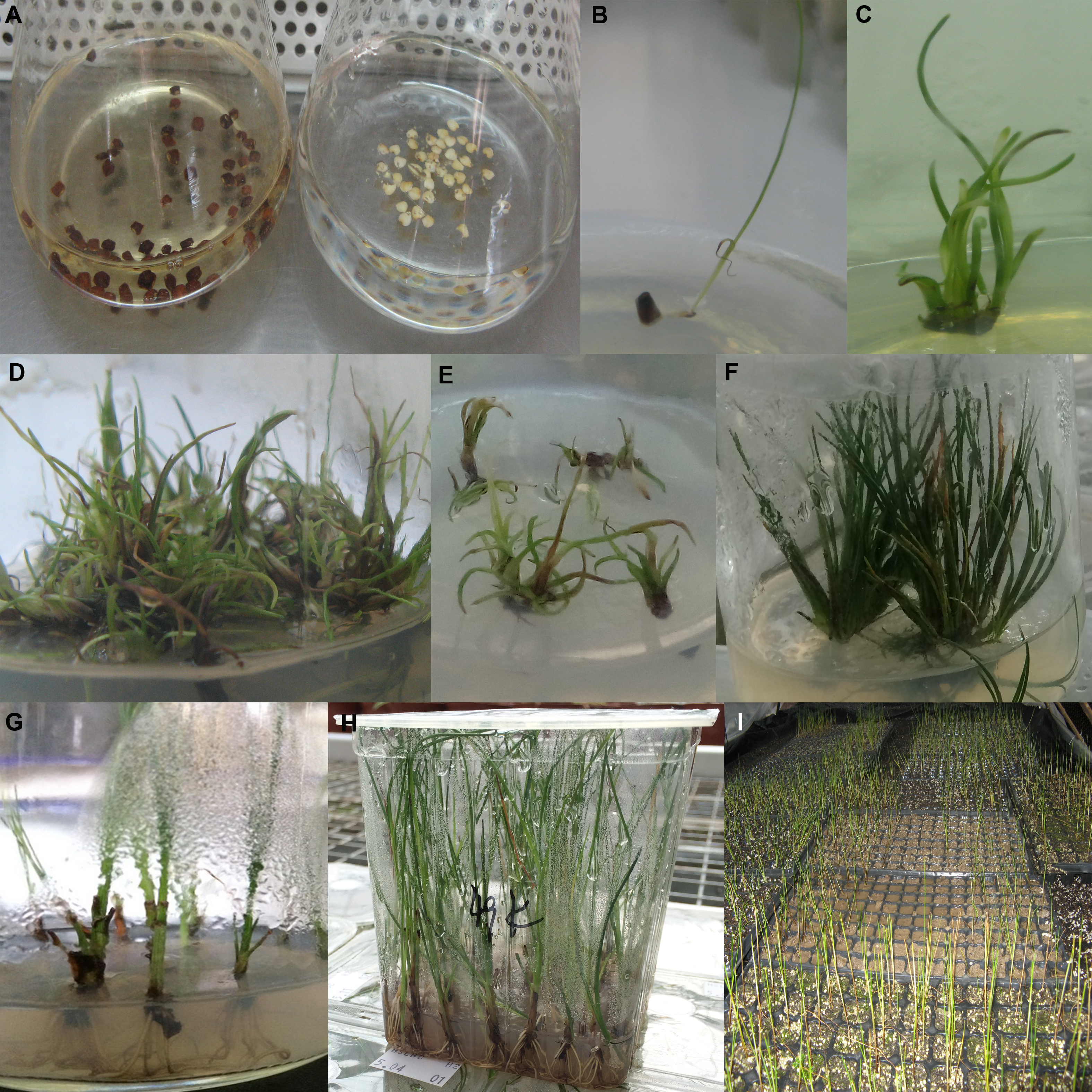
Figure 1 Different stage of plantlet regeneration from Scirpus × mariqueter seeds (A) Seed embryo partially without seed coat (left), seed embryo entirely without seed coat (right); (B) Germ-free germination seedlings; (C) Multiple shoots; (D) Multiple shoots were dwarf and cluster; (E) Dead multiple shoots under low temperature; (F) Leaves were burned by high temperature; (G) Root induction; (H) Seedlings grow in plastic container; (I) Transplanting
| No. of medium | Phytohormone composition (mg·L-1) | Multiplication coefficient | Differentiation status and color change of leaves | ||
|---|---|---|---|---|---|
| 6-BA | TDZ | IBA | |||
| M2 | 0.1 | 0 | 0.1 | 1.00d | No differentiation, yellow, weak and thin |
| M3 | 0.5 | 0 | 0.1 | 1.00d | No differentiation, yellow, weak and thin |
| M4 | 1.0 | 0 | 0.1 | 1.17d | Few differentiation, yellow-green, weak and thin |
| M5 | 2.0 | 0 | 0.2 | 1.13d | Few differentiation, weak and thin and died |
| M6 | 1.0 | 0.002 | 0.1 | 1.42c | Green, weak and thin, multiple shoots were induced |
| M7 | 1.0 | 0.002 | 0.2 | 1.50c | Green, weak and thin, multiple shoots were induced |
| M8 | 2.0 | 0.002 | 0.2 | 2.68a | Green, weak and thin, multiple shoots were induced |
| M9 | 2.0 | 0.002 | 0.3 | 2.53a | Green, weak and thin, multiple shoots were induced |
| M10 | 3.0 | 0.002 | 0.3 | 2.02b | Green, weak and thin, multiple shoots were induced |
Table 2 Effects of plant growth regulators on multiplication of Scirpus × mariqueter
| No. of medium | Phytohormone composition (mg·L-1) | Multiplication coefficient | Differentiation status and color change of leaves | ||
|---|---|---|---|---|---|
| 6-BA | TDZ | IBA | |||
| M2 | 0.1 | 0 | 0.1 | 1.00d | No differentiation, yellow, weak and thin |
| M3 | 0.5 | 0 | 0.1 | 1.00d | No differentiation, yellow, weak and thin |
| M4 | 1.0 | 0 | 0.1 | 1.17d | Few differentiation, yellow-green, weak and thin |
| M5 | 2.0 | 0 | 0.2 | 1.13d | Few differentiation, weak and thin and died |
| M6 | 1.0 | 0.002 | 0.1 | 1.42c | Green, weak and thin, multiple shoots were induced |
| M7 | 1.0 | 0.002 | 0.2 | 1.50c | Green, weak and thin, multiple shoots were induced |
| M8 | 2.0 | 0.002 | 0.2 | 2.68a | Green, weak and thin, multiple shoots were induced |
| M9 | 2.0 | 0.002 | 0.3 | 2.53a | Green, weak and thin, multiple shoots were induced |
| M10 | 3.0 | 0.002 | 0.3 | 2.02b | Green, weak and thin, multiple shoots were induced |
| No. of medium | Phytohormone composition (mg·L-1) | Multiplication coefficient | Height (cm) | Differentiation status and color change of leaves | |
|---|---|---|---|---|---|
| 6-BA | IBA | ||||
| M11 | 0 | 0 | 1.12d | 3-4 | Few yellow-green, some rooting shoots |
| M12 | 0.05 | 0.01 | 1.42cd | 3-4 | Thriving, multiple shoots uniform |
| M13 | 0.1 | 0.01 | 1.67bc | 2-3 | Relatively uniform, few small buds |
| M14 | 0.2 | 0.02 | 1.83b | 2-3 | Multiple shoots short, some small buds |
| M15 | 0.5 | 0.05 | 3.02a | 1-2 | Multiple shoots short, more small buds |
Table 4 Effects of plant growth regulators on culturing strong plants of Scirpus × mariqueter
| No. of medium | Phytohormone composition (mg·L-1) | Multiplication coefficient | Height (cm) | Differentiation status and color change of leaves | |
|---|---|---|---|---|---|
| 6-BA | IBA | ||||
| M11 | 0 | 0 | 1.12d | 3-4 | Few yellow-green, some rooting shoots |
| M12 | 0.05 | 0.01 | 1.42cd | 3-4 | Thriving, multiple shoots uniform |
| M13 | 0.1 | 0.01 | 1.67bc | 2-3 | Relatively uniform, few small buds |
| M14 | 0.2 | 0.02 | 1.83b | 2-3 | Multiple shoots short, some small buds |
| M15 | 0.5 | 0.05 | 3.02a | 1-2 | Multiple shoots short, more small buds |
| No. of medium | Phytohormone composition (mg·L-1) | Percentage of rooting shoots (%) | Description of root growing and differentiation status | |
|---|---|---|---|---|
| IBA | NAA | |||
| M16 | 0.05 | 0 | 83.33ab | Roots relatively uniform and strongly |
| M17 | 0.1 | 0 | 96.67a | Roots relatively uniform and strongly |
| M18 | 0.2 | 0 | 100.00a | Roots uniform and strongly |
| M19 | 0.5 | 0 | 100.00a | Roots uniform and too much |
| M20 | 0 | 0.2 | 66.67b | Roots not consistent, long and thin, callus were induced |
| M21 | 0.2 | 0.2 | 100.00a | Roots not consistent, long and thin, callus were induced |
Table 5 Effects of different plant growth regulators on rooting of Scirpus × mariqueter
| No. of medium | Phytohormone composition (mg·L-1) | Percentage of rooting shoots (%) | Description of root growing and differentiation status | |
|---|---|---|---|---|
| IBA | NAA | |||
| M16 | 0.05 | 0 | 83.33ab | Roots relatively uniform and strongly |
| M17 | 0.1 | 0 | 96.67a | Roots relatively uniform and strongly |
| M18 | 0.2 | 0 | 100.00a | Roots uniform and strongly |
| M19 | 0.5 | 0 | 100.00a | Roots uniform and too much |
| M20 | 0 | 0.2 | 66.67b | Roots not consistent, long and thin, callus were induced |
| M21 | 0.2 | 0.2 | 100.00a | Roots not consistent, long and thin, callus were induced |
| Culture temperature (°C) | Multiplication coefficient | Differentiation status and color change of leaves |
|---|---|---|
| 23 | 1.87d | Yellow-green and died |
| 25 | 2.68c | Green, weak and thin |
| 28 | 2.92abc | Green |
| 30 | 3.25ab | Dark green, thriving |
| 32 | 3.18a | Dark green, few burned by high temperature |
Table 3 Effects of culture temperature on multiplication of Scirpus × mariqueter
| Culture temperature (°C) | Multiplication coefficient | Differentiation status and color change of leaves |
|---|---|---|
| 23 | 1.87d | Yellow-green and died |
| 25 | 2.68c | Green, weak and thin |
| 28 | 2.92abc | Green |
| 30 | 3.25ab | Dark green, thriving |
| 32 | 3.18a | Dark green, few burned by high temperature |
| Type of container | Inoculation speed (plant·h-1) | Inoculation density (plant·container-1) | Percentage of contamination (%) | Description of root growing |
|---|---|---|---|---|
| Plastic case | 198.67 | 10 | 5.04a | Grew strongly and good condition |
| Glass bottle | 134.33 | 25 | 5.27a | Grew strongly and good condition |
Table 6 Effects of different containers on operating efficiency of Scirpus × mariqueter
| Type of container | Inoculation speed (plant·h-1) | Inoculation density (plant·container-1) | Percentage of contamination (%) | Description of root growing |
|---|---|---|---|---|
| Plastic case | 198.67 | 10 | 5.04a | Grew strongly and good condition |
| Glass bottle | 134.33 | 25 | 5.27a | Grew strongly and good condition |
| Treatment | Medium and ratio (v/v) | Density (plant·hole-1) | No. of seedlings | Survival rate (%) |
|---|---|---|---|---|
| 1 | Perlite:turf=2:1 | 1 | 60 | 31.67h |
| 2 | Perlite:turf=2:1 | 2 | 60 | 36.11gh |
| 3 | Perlite:turf=1:1 | 1 | 60 | 43.33fg |
| 4 | Perlite:turf=1:1 | 2 | 60 | 47.78ef |
| 5 | Perlite:turf=1:2 | 1 | 60 | 76.67b |
| 6 | Perlite:turf=1:2 | 2 | 60 | 69.44b |
| 7 | Perlite:turf:vermiculite=1:1:1 | 1 | 60 | 88.89a |
| 8 | Perlite:turf:vermiculite=1:1:1 | 2 | 60 | 87.22a |
| 9 | Perlite:Chongming fresh soil=1:1 | 1 | 60 | 58.33cd |
| 10 | Perlite:Chongming fresh soil=1:1 | 2 | 60 | 60.00cd |
| 11 | Chongming fresh soil | 1 | 60 | 52.78de |
| 12 | Chongming fresh soil | 2 | 60 | 61.67c |
Table 7 Effects of different medium and planting density on transplant and survival of Scirpus × mariqueter
| Treatment | Medium and ratio (v/v) | Density (plant·hole-1) | No. of seedlings | Survival rate (%) |
|---|---|---|---|---|
| 1 | Perlite:turf=2:1 | 1 | 60 | 31.67h |
| 2 | Perlite:turf=2:1 | 2 | 60 | 36.11gh |
| 3 | Perlite:turf=1:1 | 1 | 60 | 43.33fg |
| 4 | Perlite:turf=1:1 | 2 | 60 | 47.78ef |
| 5 | Perlite:turf=1:2 | 1 | 60 | 76.67b |
| 6 | Perlite:turf=1:2 | 2 | 60 | 69.44b |
| 7 | Perlite:turf:vermiculite=1:1:1 | 1 | 60 | 88.89a |
| 8 | Perlite:turf:vermiculite=1:1:1 | 2 | 60 | 87.22a |
| 9 | Perlite:Chongming fresh soil=1:1 | 1 | 60 | 58.33cd |
| 10 | Perlite:Chongming fresh soil=1:1 | 2 | 60 | 60.00cd |
| 11 | Chongming fresh soil | 1 | 60 | 52.78de |
| 12 | Chongming fresh soil | 2 | 60 | 61.67c |
| 1 | 陈建林 (2003). 荸荠组培苗高效扩繁技术的研究. 硕士论文. 扬州: 扬州大学. pp. 1-6. |
| 2 | 陈中义 (2004). 互花米草入侵国际重要湿地崇明东滩的生态后果. 博士论文. 上海: 复旦大学. pp. 2-8. |
| 3 | 陈中义 (2005). 长江口海三棱藨草的生态价值及利用与保护. 河南科技大学学报(自然科学版) 26(2) , 64-67. |
| 4 | 杜雪玲, 张振霞, 余如刚, 符义坤 (2005). 植物组织培养中的污染成因及其预防. 草业科学 22, 24-27. |
| 5 | 何松林, 孔德政, 杨秋生, 孟中生, 王美茹, 吴建华 (2003). 组织培养容器环境因子调控技术研究进展. 河南农业大学学报 37, 25-32. |
| 6 | 黄华梅 (2009). 上海滩涂盐沼植被的分布格局和时空动态研究. 博士论文. 上海: 华东师范大学. pp. 27-39. |
| 7 | 李华 (2009). 潮间带盐沼植物的沉积动力学效应研究. 博士论文. 上海: 华东师范大学. pp. 3-28. |
| 8 | 欧尚华, 方永鑫, 周根余 (1992). 海三棱藨草种子萌发条件的初步研究. 上海师范大学学报(自然科学版) 21(增刊), 23-26. |
| 9 | 童春富, 章飞军, 陆健健 (2007). 长江口海三棱藨草带生长季大型底栖动物群落变化特征. 动物学研究 28, 640-646. |
| 10 | 徐晓峰, 黄学林 (2003). TDZ: 一种有效的植物生长调节剂. 植物学通报 20, 227-237. |
| 11 | 杨梅 (2010). 海三棱藨草的物种生物学和遗传结构研究. 博士论文. 上海: 复旦大学. pp. 8-10. |
| 12 | 赵雨云, 马志军, 陈家宽 (2002). 崇明东滩越冬白头鹤食性的研究. 复旦学报(自然科学版) 41, 609-613. |
| 13 | 周博, 许维倩, 李娜, 徐娜, 姜长阳 (2010). 银线伞莎草组织培养及快速繁殖的研究. 北方园艺 17, 150-152. |
| 14 | 朱晶, 敬凯, 干晓静, 马志军 (2007). 迁徙停歇期鸻鹬类在崇明东滩潮间带的食物分布. 生态学报 27, 2149-2159. |
| 15 | Chalupa V (1987). Effect of benzylaminopurine and thidiazuron on in vitro shoot proliferation of Tilia cordata Mill, Sorbus aucuparia L. and Robinia pseudoacacia L.Biol Plantarum 29, 425-429. |
| 16 | Chen H, Wang DQ, Chen ZL, Wang J, Xu SY (2005). The variation of sediments organic carbon content in Chong- ming east tidal flat during Scirpus mariqueter growing stage.J Geogr Sci 15, 500-508. |
| 17 | Gan XJ, Cai YT, Choi CY, Ma ZJ, Chen JK, Li B (2009). Potential impacts of invasive Spartina alterniflora on spring bird communities at Chongming Dongtan, a Chi- nese wetland of international importance.Estuar Coast Shelf S 83, 211-218. |
| 18 | Li B, Liao CH, Zhang XD, Chen HL, Wang Q, Chen ZY, Gan XJ, Wu JH, Zhao B, Ma ZJ, Cheng XL, Jiang LF, Chen JK (2009). Spartina alterniflora invasions in the Yangtze River Estuary, China: an overview of current status and ecosystem effects.Ecol Eng 35, 511-520. |
| 19 | Liao CZ, Peng RH, Luo YQ, Zhou XH, Wu XW, Fang CM, Chen JK, Li B (2008). Altered ecosystem carbon and nitrogen cycles by plant invasion: a meta-analysis.New Phytol 177, 706-714. |
| 20 | Peng RH, Fang CM, Li B, Chen JK (2011). Spartina al- terniflora invasion increases soil inorganic nitrogen pools through interactions with tidal subsidies in the Yangtze Estuary, China.Oecologia 165, 797-807. |
| 21 | Quan WM, Fu CZ, Jin BS, Luo YQ, Li B, Chen JK, Wu JH (2007). Tidal marshes as energy sources for commercially important nektonic organisms: stable isotope analysis.Mar Ecol Prog Ser 352, 89-99. |
| 22 | Wang M, Chen JK, Li B (2007). Characterization of bacterial community structure and diversity in rhizosphere soils of three plants in rapidly changing salt marshes using 16S rDNA.Pedosphere 17, 545-556. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||