

植物学报 ›› 2015, Vol. 50 ›› Issue (3): 394-404.DOI: 10.3724/SP.J.1259.2015.00394
• 专题论坛 • 上一篇
薛轶群1, 宋凯1, 范路生1, 万迎朗2, 林金星1,2,*( )
)
收稿日期:2014-06-04
接受日期:2014-09-09
出版日期:2015-05-01
发布日期:2015-04-08
通讯作者:
林金星
作者简介:? 共同第一作者
基金资助:Yiqun Xue1, Kai Song1, Lusheng Fan1, Yinglang Wan2, Jinxing Lin1, 2, *
Received:2014-06-04
Accepted:2014-09-09
Online:2015-05-01
Published:2015-04-08
Contact:
Lin Jinxing
About author:? These authors contributed equally to this paper
摘要: pH敏感型荧光蛋白, 即pHluorin, 是荧光强度及光谱特征随环境pH值的变化而改变的一类荧光蛋白。人们通过对密码子使用偏好和特定剪切位点的修饰, 已使pHluorin及其衍生物成功地在动物、植物和真菌细胞中正常表达, 为测量细胞内微环境pH值的变化, 并研究活细胞内依赖或导致pH变化的生理过程提供了有力工具。该文总结了目前已报道的pH敏感型荧光蛋白的种类及特性, 并对其在细胞生物学, 特别是植物细胞生物学中的应用进行了详细介绍。随着报告基因技术及检测方法的不断改进, pHluorin将在植物科学领域发挥更大的作用。
薛轶群, 宋凯, 范路生, 万迎朗, 林金星. pH敏感型荧光蛋白及其在植物细胞生物学中的应用. 植物学报, 2015, 50(3): 394-404.
Yiqun Xue, Kai Song, Lusheng Fan, Yinglang Wan, Jinxing Lin. pH-sensitive Fluorescent Proteins and Their Applications in Plant Cell Biology. Chinese Bulletin of Botany, 2015, 50(3): 394-404.
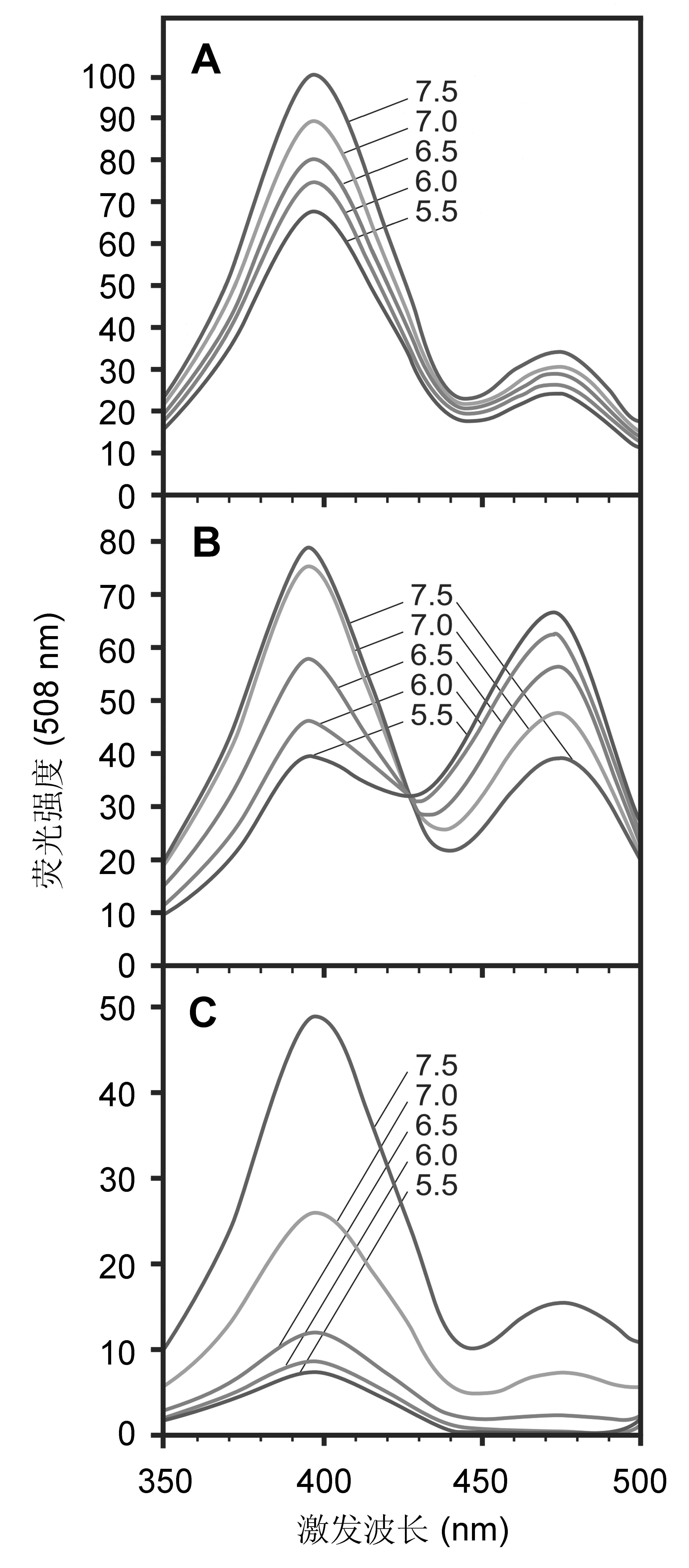
图1 GFP及pHluorin的荧光激发光谱(Miesenböck et al., 1998) (A) 野生型GFP; (B) 比率pHluorin; (C) 盈缺pHluorin
Figure 1 Fluorescence excitation spectra (Miesenböck et al., 1998) (A) Wild-type GFP; (B) Ratiometric pHluorin; (C) Ecliptic pHluorin
| 荧光蛋白名称 | 类型 | 激发光(nm) | 发射光(nm) | pH范围 | pKa | 目前应用范畴 | 参考文献 |
|---|---|---|---|---|---|---|---|
| pHluorin | 比率 | 395/475 | 510 | 5.4-8.4 | 6.9 | 测量动物细胞内涵体和TGN中pH值 | Miesenböck et al., 1998 |
| pHluorin2 | 比率 | 395/475 | 510 | 5.4-8.4 | 6.9 | 检测动物细胞中激素受体内吞 | Mahon, 2011 |
| RaVC | 比率 | 395/475 | 510 | 5.4-8.4 | 6.9 | 测量曲霉菌中胞质pH值 | Bagar et al., 2009 |
| pHGFP | 比率 | 395/475 | 510 | 5.4-8.4 | 6.9 | 测量拟南芥根中胞质pH值 | Moseyko and Feldman, 2001 |
| Ratiometric AtpHluorin | 比率 | 395/475 | 510 | 5.4-8.4 | 6.9 | 测量拟南芥根质外体和细胞质中pH值 | Gao et al., 2004 |
| PRpHluorin | 比率 | 395/475 | 510 | 5.4-8.4 | 6.9 | 测量拟南芥原生质体中细胞器pH值 | Shen et al., 2013 |
| Ecliptic pHluorin | 盈缺 | 395/477 | 510 | 6.5-8.0 | 7.2 | 检测动物细胞突触囊泡胞吐 | Miesenböck et al., 1998 |
| Ecliptic AtpHluorin | 盈缺 | 395/477 | 510 | 6.5-8.0 | 7.2 | 测量拟南芥根质外体和细胞质中pH值 | Gao et al., 2004 |
| PEpHluorin | 盈缺 | 395/477 | 510 | 6.5-8.0 | 7.2 | 测量拟南芥原生质体中细胞器pH值 | Shen et al., 2013 |
| Superecliptic pHluorin | 盈缺 | 477 | 510 | 5.5-8.5 | 7.2 | 检测动物细胞突触末端胞吞胞吐 | Sankaranarayanan and Ryan, 2001 |
| deGFP | 比率 | 396 | 460/515 | 6-9.0 | 7.3 | 测量动物细胞中pH值 | Hanson et al., 2002 |
| E2GFP | 比率 | 458/488 | 500/560 | 5-8.5 | 6.9/7.5 | 测量动物细胞中pH值 | Bizzarri et al., 2006; Arosio et al., 2007 |
| ptGFP | 比率 | 390/475 | 540 | 3.8-8.2 | 7.3 | 测量拟南芥根质外体和细胞质中pH值 | Schulte et al., 2006 |
| pHlameleon 6 | 比率 | 458 | 481/533 | 5-7.5 | 5.6 | 基于FRET原理测量动物细胞中pH值 | Esposito et al., 2008 |
| pHusion | 比率 | 488/458 | 510/600 | 4.5-8 | 5.8 | 基于FRET原理测量拟南芥叶肉和根 的固定细胞中质外体和胞质pH值 | Gjetting et al., 2012 |
| pHlash | 比率 | 不适用 | 475/525 | 5.4-9 | 不适用 | 基于BRET原理测量动物细胞中胞质 pH值 | Zhang et al., 2012 |
表1 部分pH敏感型荧光蛋白的光谱特性及pH测量范围(改编自Martinière et al., 2013a)
Table 1 Spectral character of selected pH-sensitive fluorescence proteins and their pH measurement range (adapted from Martinière et al., 2013a)
| 荧光蛋白名称 | 类型 | 激发光(nm) | 发射光(nm) | pH范围 | pKa | 目前应用范畴 | 参考文献 |
|---|---|---|---|---|---|---|---|
| pHluorin | 比率 | 395/475 | 510 | 5.4-8.4 | 6.9 | 测量动物细胞内涵体和TGN中pH值 | Miesenböck et al., 1998 |
| pHluorin2 | 比率 | 395/475 | 510 | 5.4-8.4 | 6.9 | 检测动物细胞中激素受体内吞 | Mahon, 2011 |
| RaVC | 比率 | 395/475 | 510 | 5.4-8.4 | 6.9 | 测量曲霉菌中胞质pH值 | Bagar et al., 2009 |
| pHGFP | 比率 | 395/475 | 510 | 5.4-8.4 | 6.9 | 测量拟南芥根中胞质pH值 | Moseyko and Feldman, 2001 |
| Ratiometric AtpHluorin | 比率 | 395/475 | 510 | 5.4-8.4 | 6.9 | 测量拟南芥根质外体和细胞质中pH值 | Gao et al., 2004 |
| PRpHluorin | 比率 | 395/475 | 510 | 5.4-8.4 | 6.9 | 测量拟南芥原生质体中细胞器pH值 | Shen et al., 2013 |
| Ecliptic pHluorin | 盈缺 | 395/477 | 510 | 6.5-8.0 | 7.2 | 检测动物细胞突触囊泡胞吐 | Miesenböck et al., 1998 |
| Ecliptic AtpHluorin | 盈缺 | 395/477 | 510 | 6.5-8.0 | 7.2 | 测量拟南芥根质外体和细胞质中pH值 | Gao et al., 2004 |
| PEpHluorin | 盈缺 | 395/477 | 510 | 6.5-8.0 | 7.2 | 测量拟南芥原生质体中细胞器pH值 | Shen et al., 2013 |
| Superecliptic pHluorin | 盈缺 | 477 | 510 | 5.5-8.5 | 7.2 | 检测动物细胞突触末端胞吞胞吐 | Sankaranarayanan and Ryan, 2001 |
| deGFP | 比率 | 396 | 460/515 | 6-9.0 | 7.3 | 测量动物细胞中pH值 | Hanson et al., 2002 |
| E2GFP | 比率 | 458/488 | 500/560 | 5-8.5 | 6.9/7.5 | 测量动物细胞中pH值 | Bizzarri et al., 2006; Arosio et al., 2007 |
| ptGFP | 比率 | 390/475 | 540 | 3.8-8.2 | 7.3 | 测量拟南芥根质外体和细胞质中pH值 | Schulte et al., 2006 |
| pHlameleon 6 | 比率 | 458 | 481/533 | 5-7.5 | 5.6 | 基于FRET原理测量动物细胞中pH值 | Esposito et al., 2008 |
| pHusion | 比率 | 488/458 | 510/600 | 4.5-8 | 5.8 | 基于FRET原理测量拟南芥叶肉和根 的固定细胞中质外体和胞质pH值 | Gjetting et al., 2012 |
| pHlash | 比率 | 不适用 | 475/525 | 5.4-9 | 不适用 | 基于BRET原理测量动物细胞中胞质 pH值 | Zhang et al., 2012 |
| 1 | 邓超, 黄大昉, 宋福平 (2011). 绿色荧光蛋白及其应用. 中国生物工程杂志 31, 96-102. |
| 2 | 黄国存, 朱生伟, 董越梅, 孙敬三 (1998). 绿色荧光蛋白及其在植物研究中的应用. 植物学通报 15, 24-30. |
| 3 | 李晓娟, 王钦丽, 苏月, 林金星 (2009). 量子点标记及其在植物细胞生物学中的应用. 电子显微学报 28, 495-504. |
| 4 | 王锋, 张春雨, 万里川, 王钦丽, 林金星 (2010). 光激活荧光蛋白及其在植物分子细胞生物学研究中的应用. 植物学报 45, 530-540. |
| 5 | 周围, 朱丹, 梁滔, 李臣鸿, 吴政星 (2007). 利用TIRFM技术检测PC12细胞中类突触小囊泡的锚定和融合特性. 科学通报 52, 2276-2282. |
| 6 | Arosio D, Garau G, Ricci F, Marchetti L, Bizzarri R, Nifosì R, Beltram F (2007). Spectroscopic and structural study of proton and halide ion cooperative binding to GFP.Biophys J 93, 232-244. |
| 7 | Ashby MC, Ibaraki K, Henley JM (2004). It’s green outside: tracking cell surface proteins with pH-sensitive GFP.Trends Neurosci 27, 257-261. |
| 8 | Bagar T, Altenbach K, Read ND, Benčina M (2009). Live-cell imaging and measurement of intracellular pH in filamentous fungi using a genetically encoded ratiometric probe.Eukaryot Cell 8, 703-712. |
| 9 | Bassil E, Ohto MA, Esumi T, Tajima H, Zhu Z, Cagnac O, Belmonte M, Peleg Z, Yamaguchi T, Blumwald E (2011). The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and neces- sary for plant growth and development.Plant Cell 23, 224-239. |
| 10 | Bizzarri R, Arcangeli C, Arosio D, Ricci F, Faraci P, Cardarelli F, Beltram F (2006). Development of a novel GFP-based ratiometric excitation and emission pH indi- cator for intracellular studies.Biophys J 90, 3300-3314. |
| 11 | Bizzarri R, Serresi M, Luin S, Beltram F (2009). Green fluorescent protein based pH indicators for in vivo use: a review.Anal Bioanal Chem 393, 1107-1122. |
| 12 | Demaurex N (2002). pH Homeostasis of cellular organelles. News Physiol Sci 17, 1-5. |
| 13 | Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schuma- cher K (2006). Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis.Plant Cell 18, 715-730. |
| 14 | Esposito A, Gralle M, Dani MAC, Lange D, Wouters FS (2008). pHlameleons: a family of FRET-based protein sensors for quantitative pH imaging.Biochemistry 47, 13115-13126. |
| 15 | Felle HH (1998). The apoplastic pH of the Zea mays root cortex as measured with pH-sensitive microelectrodes: aspects of regulation.J Exp Bot 49, 987-995. |
| 16 | Fricker M, Runions J, Moore I (2006). Quantitative fluorescence microscopy: from art to science.Annu Rev Plant Biol 57, 79-107. |
| 17 | Gao DJ, Knight MR, Trewavas AJ, Sattelmacher B, Plieth C (2004). Self-reporting Arabidopsis expressing pH and [Ca2+] indicators unveil ion dynamics in the cytoplasm and in the apoplast under abiotic stress.Plant Physiol 134, 898-908. |
| 18 | Gehring CA, Irving HR, McConchie R, Parish RW (1997). Jasmonates induce intracellular alkalinization and closure of Paphiopedilum guard cells.Ann Bot 80, 485-489. |
| 19 | Gjetting KS, Ytting CK, Schulz A, Fuglsang AT (2012). Live imaging of intra- and extracellular pH in plants using pHusion, a novel genetically encoded biosensor.J Exp Bot 63, 3207-3218. |
| 20 | Gonugunta VK, Srivastava N, Puli MR, Raghavendra AS (2008). Nitric oxide production occurs after cytosolic alkalinization during stomatal closure induced by abscisic acid.Plant Cell Environ 31, 1717-1724. |
| 21 | Grignon C, Sentenac H (1991). pH and ionic conditions in the apoplast.Annu Rev Plant Physiol Plant Mol Biol 42, 103-128. |
| 22 | Hanna ST, Pigeau GM, Galvanovskis J, Clark A, Rorsman P, MacDonald PE (2009). Kiss-and-run exocytosis and fusion pores of secretory vesicles in human β-cells. Pflugers Arch 457, 1343-1350. |
| 23 | Hanson GT, McAnaney TB, Park ES, Rendell MEP, Yarbrough DK, Chu SY, Xi LX, Boxer SG, Montrose MH, Remington SJ (2002). Green fluorescent protein variants as ratiometric dual emission pH sensors. 1. Structural characterization and preliminary application.Biochemistry 41, 15477-15488. |
| 24 | Hoffmann B, Kosegarten H (1995). FITC-dextran for measuring apoplast pH and apoplastic pH gradients bet- ween various cell types in sunflower leaves.Physiol Plant 95, 327-335. |
| 25 | Jankowski A, Kim JH, Collins RF, Daneman R, Walton P, Grinstein S (2001). In situ measurements of the pH of mammalian peroxisomes using the fluorescent protein pHluorin.J Biol Chem 276, 48748-48753. |
| 26 | Jin T, Sasaki A, Kinjo M, Miyazaki J (2010). A quantum dot-based ratiometric pH sensor.Chem Commun 46, 2408-2410. |
| 27 | Karagiannis J, Young PG (2001). Intracellular pH homeo- stasis during cell-cycle progression and growth state transition in Schizosaccharomyces pombe. J Cell Sci 114, 2929-2941. |
| 28 | Kirsch T, Paris N, Butler JM, Beevers L, Rogers JC (1994). Purification and initial characterization of a potential plant vacuolar targeting receptor.Proc Natl Acad Sci USA 91, 3403-3407. |
| 29 | Kneen M, Farinas J, Li YX, Verkman AS (1998). Green fluorescent protein as a noninvasive intracellular pH indicator.Biophys J 74, 1591-1599. |
| 30 | Kononenko NL, Diril MK, Puchkov D, Kintscher M, Koo SJ, Pfuhl G, Winter Y, Wienisch M, Klingauf J, Breustedt J, Schmitz D, Maritzen T, Haucke V (2013). Compromised fidelity of endocytic synaptic vesicle protein sorting in the absence of stonin 2.Proc Natl Acad Sci USA 110, E526-E535. |
| 31 | Krebs M, Beyhl D, Görlich E, Al-Rasheid KAS, Marten I, Stierhof YD, Hedrich R, Schumacher K (2010). Arabi- dopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation.Proc Natl Acad Sci USA 107, 3251-3256. |
| 32 | Li ZY, Murthy VN (2001). Visualizing postendocytic traffic of synaptic vesicles at hippocampal synapses.Neuron 31, 593-605. |
| 33 | Lippincott-Schwartz J, Roberts TH, Hirschberg K (2000). Secretory protein trafficking and organelle dynamics in living cells.Annu Rev Cell Dev Biol 16, 557-589. |
| 34 | Lukyanov KA, Chudakov DM, Lukyanov S, Verkhusha VV (2005). Innovation: photoactivatable fluorescent pro- teins.Nat Rev Mol Cell Biol 6, 885-890. |
| 35 | Machen TE, Leigh MJ, Taylor C, Kimura T, Asano S, Moore HP (2003). pH of TGN and recycling endosomes of H+/K+-ATPase-transfected HEK-293 cells: implications for pH regulation in the secretory pathway.Am J Physiol Cell Physiol 285, C205-C214. |
| 36 | Mahon MJ (2011). pHluorin2: an enhanced, ratiometric, pH-sensitive green florescent protein.Adv Biosci Biotechnol 2, 132-137. |
| 37 | Malinow R, Malenka RC (2002). AMPA receptor traffic- king and synaptic plasticity.Annu Rev Neurosci 25, 103-126. |
| 38 | Martinière A, Desbrosses G, Sentenac H, Paris N (2013a). Development and properties of genetically encoded pH sensors in plants.Front Plant Sci 4, 523. |
| 39 | Martinière A, Bassil E, Jublanc E, Alcon C, Reguera M, Sentenac H, Blumwald E, Paris N (2013b). In vivo in- tracellular pH measurements in tobacco and Arabidopsis reveal an unexpected pH gradient in the endomembrane system.Plant Cell 25, 4028-4043. |
| 40 | Miesenböck G, De Angelis DA, Rothman JE (1998). Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins.Nature 394, 192-195. |
| 41 | Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY (1997). Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin.Nature 388, 882-887. |
| 42 | Monshausen GB, Miller ND, Murphy AS, Gilroy S (2011). Dynamics of auxin-dependent Ca2+ and pH signaling in root growth revealed by integrating high-resolution imaging with automated computer vision-based analysis.Plant J 65, 309-318. |
| 43 | Moseyko N, Feldman LJ (2001). Expression of pH-sensitive green fluorescent protein in Arabidopsis thaliana.Plant Cell Environ 24, 557-563. |
| 44 | Mühling KH, Sattelmacher B (1995). Apoplastic ion con- centration of intact leaves of field bean (Vicia faba) as influenced by ammonium and nitrate nutrition.J Plant Physiol 147, 81-86. |
| 45 | Nicholson-Tomishima K, Ryan TA (2004). Kinetic effi- ciency of endocytosis at mammalian CNS synapses requires synaptotagmin I.Proc Natl Acad Sci USA 101, 16648-16652. |
| 46 | Ohara-Imaizumi M, Nakamichi Y, Tanaka T, Katsuta H, Ishida H, Nagamatsu S (2002). Monitoring of exocytosis and endocytosis of insulin secretory granules in the pancreatic beta-cell line MIN6 using pH-sensitive green fluorescent protein (pHluorin) and confocal laser microscopy.Biochem J 363, 73-80. |
| 47 | Orij R, Postmus J, Ter Beek A, Brul S, Smits GJ (2009). In vivo measurement of cytosolic and mitochondrial pH using a pH-sensitive GFP derivative in Saccharomyces ce- revisiae reveals a relation between intracellular pH and growth.Microbiology 155, 268-278. |
| 48 | Prosser DC, Whitworth K, Wendland B (2010). Quantitative analysis of endocytosis with cytoplasmic pHluorin chimeras.Traffic 11, 1141-1150. |
| 49 | Quintero FJ, Martinez-Atienza J, Villalta I, Jiang XY, Kim WY, Ali Z, Fujii H, Mendoza I, Yun DJ, Zhu JK, Pardo JM (2011). Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain.Proc Natl Acad Sci USA 108, 2611-2616. |
| 50 | Rayle DL, Cleland RE (1992). The Acid Growth Theory of auxin-induced cell elongation is alive and well.Plant Physiol 99, 1271-1274. |
| 51 | Robinson DG, Pimpl P (2014). Receptor-mediated trans- port of vacuolar proteins: a critical analysis and a new model.Protoplasma 251, 247-264. |
| 52 | Robinson DG, Pimpl P, Scheuring D, Stierhof YD, Sturm S, Viotti C (2012). Trying to make sense of retromer.Trends Plant Sci 17, 431-439. |
| 53 | Saint-Jean B, Seveno-Carpentier E, Alcon C, Neuhaus JM, Paris N (2010). The cytosolic tail dipeptide Ile-Met of the pea receptor BP80 is required for recycling from the prevacuole and for endocytosis.Plant Cell 22, 2825-2837. |
| 54 | Sankaranarayanan S, Ryan TA (2000). Real-time measu- rements of vesicle-SNARE recycling in synapses of the central nervous system. Nat Cell Biol 2, 197-204. |
| 55 | Sankaranarayanan S, Ryan TA (2001). Calcium acceler- ates endocytosis of vSNAREs at hippocampal synapses. Nat Neurosci 4, 129-136. |
| 56 | Schulte A, Lorenzen I, Böttcher M, Plieth C (2006). A novel fluorescent pH probe for expression in plants.Plant Methods 2, 7. |
| 57 | Sentenac H, Grignon C (1987). Effect of H+ excretion on the surface pH of corn root cells evaluated by using weak acid influx as a pH probe.Plant Physiol 84, 1367-1372. |
| 58 | Shaner NC, Campbell RE, Steinbach PA, Giepmans BN, Palmer AE, Tsien RY (2004). Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein.Nat Biotechnol 22, 1567-1572. |
| 59 | Shen JB, Zeng YL, Zhuang XH, Sun L, Yao XQ, Pimpl P, Jiang LW (2013). Organelle pH in the Arabidopsis endomembrane system.Mol Plant 6, 1419-1437. |
| 60 | Shimomura O, Johnson FH, Saiga Y (1962). Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan,Aequorea. J Cell Comp Physiol 59, 223-239. |
| 61 | Staal M, De Cnodder T, Simon D, Vandenbussche F, Van der Straeten D, Verbelen JP, Elzenga T, Vissenberg K (2011). Apoplastic alkalinization is instrumental for the inhibition of cell elongation in the Arabidopsis root by the ethy- lene precursor 1-aminocyclopropane-1-carboxylic acid.Pl- ant Physiol 155, 2049-2055. |
| 62 | Taylor DP, Slattery J, Leopold AC (1996). Apoplastic pH in corn root gravitropism: a laser scanning confocal microscopy measurement.Physiol Plant 97, 35-38. |
| 63 | Terskikh A, Fradkov A, Ermakova G, Zaraisky A, Tan P, Kajava AV, Zhao XN, Lukyanov S, Matz M, Kim S, Weissman I, Siebert P (2000). “Fluorescent timer”: protein that changes color with time.Science 290, 1585-1588. |
| 64 | Thibaud JB, Davidian JC, Sentenac H, Soler A, Grignon C (1988). H+ cotransports in corn roots as related to the surface pH shift induced by active H+ excretion.Plant Physiol 88, 1469-1473. |
| 65 | Toulon V, Sentenac H, Thibaud JB, Davidian JC, Moulineau C, Grignon C (1992). Role of apoplast acidification by the H+ pump: effect on the sensitivity to pH and CO2 of iron reduction by roots of Brassica napus L.Planta 186, 212-218. |
| 66 | Toulon V, Sentenac H, Thibaud JB, Soler A, Clarkson D, Grignon C (1989). Effect of HCO3- concentration in the absorption solution on the energetic coupling of H+-co- transports in roots of Zea mays L.Planta 179, 235-241. |
| 67 | Tsien RY (2005). Building and breeding molecules to spy on cells and tumors.FEBS Lett 579, 927-932. |
| 68 | Tsuboi T, Rutter GA (2003). Multiple forms of “kiss-and-run” exocytosis revealed by evanescent wave microscopy.Curr Biol 13, 563-567. |
| 69 | Wachter RM (2007). Chromogenic cross-link formation in green fluorescent protein.Acc Chem Res 40, 120-127. |
| 70 | Wan YL, Ash WM 3rd, Fan LS, Hao HQ, Kim MK, Lin JX (2011). Variable-angle total internal reflection fluores- cence microscopy of intact cells of Arabidopsis thaliana.Plant Methods 7, 27. |
| 71 | Wang QL, Zhao YY, Luo WX, Li RL, He QH, Fang XH, De Michele R, Ast C, von Wirén N, Lin JX (2013). Single-particle analysis reveals shutoff control of the Arabidopsis ammonium transporter AMT1;3 by clustering and internalization.Proc Natl Acad Sci USA 110, 13204-13209. |
| 72 | Yang TT, Cheng LZ, Kain SR (1996). Optimized codon usage and chromophore mutations provide enhanced sensitivity with the green fluorescent protein.Nucleic Aci- ds Res 24, 4592-4593. |
| 73 | Zhang YF, Xie QG, Robertson JB, Johnson CH (2012). pHlash: a new genetically encoded and ratiometric lumi- nescence sensor of intracellular pH.PLoS One 7, e43072. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||